If you are interested in participating in a Clinical Research Trial, please fill out this form or click the “learn more” button. A representative will contact you within 24 business hours with more information .

Clinical trials are research studies designed to evaluate new medical, surgical, or behavioral interventions in order to answer specific health questions.
Most clinical trials are used to determine if a new treatment is more effective and/or has less harmful side effects than the current, standard treatment. Other clinical trials test early detection methods, investigate ways to prevent a health problem all together, or explore how to make life better for people living with a life-threatening disease or chronic health problem.
Regardless of the reason, clinical trials are the safest and fastest way to find new treatments and improve the health of society.
Clinical trials play a critical role in the advancement of medicine. Clinical trials are vital for lengthening life, enhancing health, and reducing the burdens of disability and illness.
Clinical trials are responsible for finding an effective treatment for significant illnesses and diseases as well as discovering early illness detection through cutting-edge studies.
Without clinical trials, many of our lifesaving medicines, devices, and treatments would not exist today.
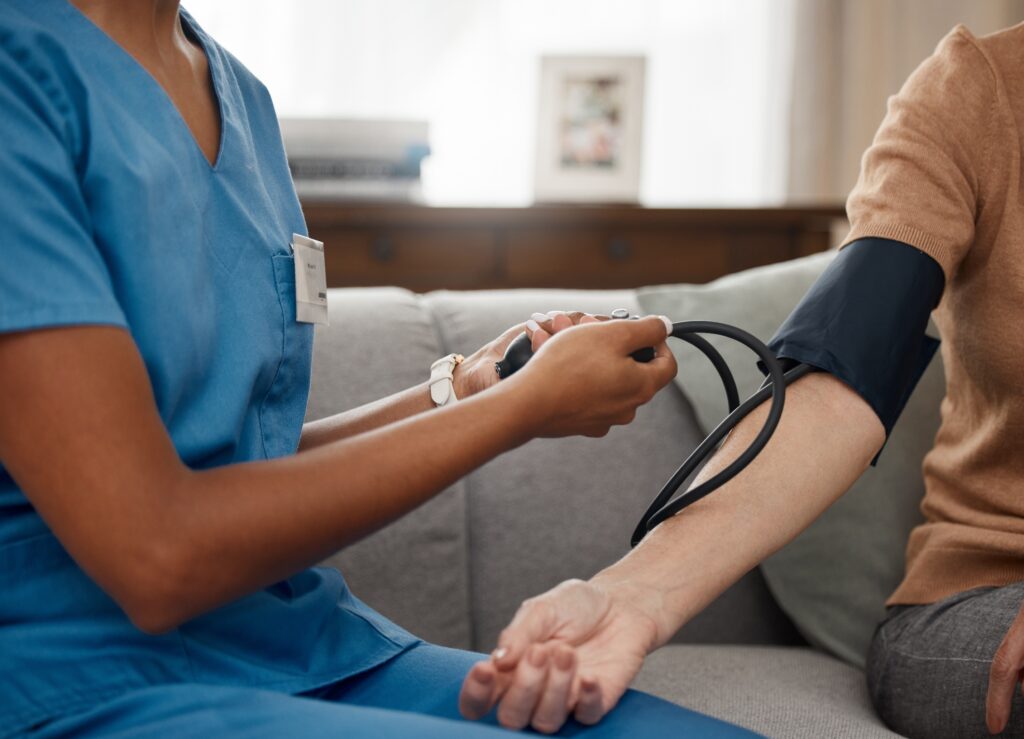
Many participants volunteer in clinical trials with the hopes that this new medicine or therapy will improve their condition and quality of life. This may be especially important for volunteers who have not had success with the current treatments available.
Participants may volunteer in memory of a loved one or in hopes that new, cutting-edge therapies may benefit their loved ones down the line. In these cases, volunteers may be drawn to studies on disabilities or illnesses that run in their families.
Some volunteers participate in clinical trials to pave the way forward towards better health for future generations. They often feel passionate about assisting in the discovery of new and effective medial approaches.
If you have more questions regarding the process of participating in a clinical research trial, please read below to find out how you can be matched to a study that is right for you. You may also visit our FAQ section below if you have further questions.

*Scroll down to find out more about the Informed Consent Form.
If you are interested in participating in a Clinical Research Trial, please fill out this form or click the “learn more” button. A representative will contact you within 24 business hours with more information .
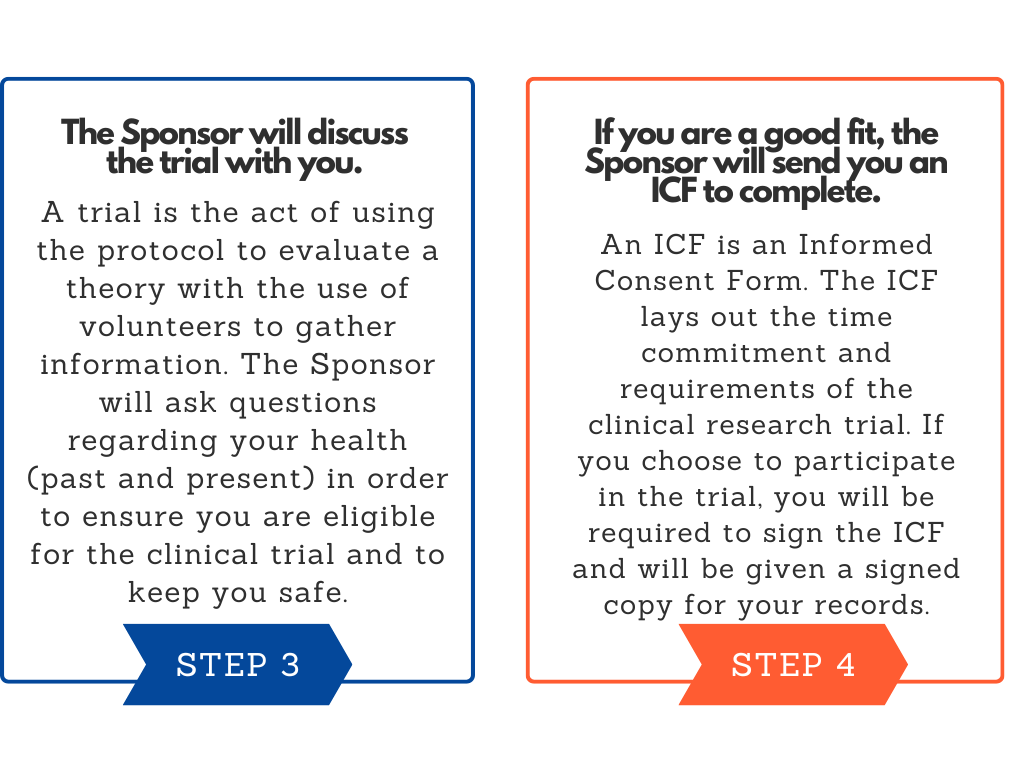
The Sponsor will ask questions regarding your health (past and present) in order to ensure you are eligible for the clinical trial and to keep you safe.
Clinical trials are conducted to see if a new drug, treatment, device, process or a combination of these can improve a person’s quality of life.
The trial Sponsors will help you find a place that is convenient for you to visit and complete the trial in your new location.
Absolutely not! If it is a trial requirement the sponsors of the trial are required to pay for it.
Often trial sponsors will provide a stipend to pay for your time and travel.
The consent form will provide contact information for you regarding any questions about the trial or any formal complaint you wish to launch.
The study staff (MD, Nurse, Social worker) will let you know you have finished.
The study staff are required to explain all risks when they review the consent form with you.
A “placebo” is a substance designed to look like the medication being tested, but it is not active. Using a placebo in this way can help prevent patients and their doctors from figuring out which treatment group they were assigned to which helps prevent bias in clinical research.
Probably not. Especially if it is a blinded study since the study staff won’t know either.
If you have great success while in the trial, the trial MD will help you find a suitable alternative.
They can contact the study office at any time to speak to the study MD regarding the trial and your participation.
The ICF (Informed Consent Form) is an important document that explains the time commitment and requirements of the Clinical Research Trial one may be interested in participating in.
You are encouraged to read the ICF (Informed Consent Form) front to back. You may also choose to discuss it with a trusted healthcare professional or send a copy to your MD if you have any questions. The ICF will provide the length of time you are required to participate with the number of study visits and procedures during those visits.
Below are a few examples of what may appear on an ICF and how they may impact you.
If you choose to participate in a clinical trial, you will be required to sign the ICF and will be given a signed copy for your records.
You are a volunteer, so that is your right; however, once you have taken an investigational drug it is important to let the study doctor continue to follow you for safety purposes.
That is the name of the protocol. An example might look like: Protocol Number xxxx-xxxx, Phase III, Pragmatic Randomized Blinded Clinical Trial in a Community-Based Setting Comparing xxx + xxx vs. xxxx in Patients with xxxx.
A protocol number is the number assigned to the plan. Sponsors may have many trials being conducted at one time. The protocol number helps them identify each project.
This number is assigned by the Sponsor and is registered with the FDA. You can look up any study on the government website: www.clinicaltrials.gov
A “Phase” refers to the part of the life cycle of testing. A protocol typically has 3 phases to complete before the FDA will evaluate the information gathered and recommend it for marketing.
Randomized is a way to assign each subject to a specific drug schedule within the trial.
“Blinded” means the person giving you the medication does not and cannot know the dose you have been given, or if your medication is a placebo.
If you are interested in participating in a Clinical Research Trial, please fill out this form or click the “learn more” button. A representative will contact you within 24 business hours with more information .